<< Previous page INDEX Next page >>
2.5 The Lithium Ion Battery
Pioneer work with the lithium battery began in 1912 under G.N. Lewis but it was not until the early 1970s that the first non-rechargeable lithium batteries became commercially available. Attempts to develop rechargeable lithium batteries followed in the 1980s, but failed due to safety problems.
Lithium is the lightest of all metals, has the greatest electrochemical potential and provides the largest energy density per weight. Rechargeable batteries using lithium metal anodes (negative electrodes) are capable of providing both high voltage and excellent capacity, resulting in an extraordinary high energy density.
After much research on rechargeable lithium batteries during the 1980s, it was found that cycling causes changes on the lithium electrode. These transformations, which are part of normal wear and tear, reduce the thermal stability, causing potential thermal runaway conditions. When this occurs, the cell temperature quickly approaches the melting point of lithium, resulting in a violent reaction called ‘venting with flame’. A large quantity of rechargeable lithium batteries sent to Japan had to be recalled in 1991 after a battery in a mobile phone released flaming gases and inflicted burns to a person’s face.
Because of the inherent instability of lithium metal, especially during charging, research shifted to a non-metallic lithium battery using lithium ions. Although slightly lower in energy density than lithium metal, the Li-ion is safe, provided certain precautions are met when charging and discharging. In 1991, the Sony Corporation commercialized the first Li-ion battery. Other manufacturers followed suit. Today, the Li-ion is the fastest growing and most promising battery chemistry.
The energy density of the Li-ion is typically twice that of the standard NiCd. Improvements in electrode active materials have the potential of increasing the energy density close to three times that of the NiCd. In addition to high capacity, the load characteristics are reasonably good and behave similarly to the NiCd in terms of discharge characteristics (similar shape of discharge profile, but different voltage). The flat discharge curve offers effective utilization of the stored power in a desirable voltage spectrum.
The Li-ion is a low maintenance battery, an advantage that most other chemistries cannot claim. There is no memory and no scheduled cycling is required to prolong the battery’s life. In addition, the self-discharge is less than half compared to NiCd and NiMH, making the Li-ion well suited for modern fuel gauge applications.
The high cell voltage of Li-ion allows the manufacture of battery packs consisting of only one cell. Many of today’s mobile phones run on a single cell, an advantage that simplifies battery design. Supply voltages of electronic applications have been heading lower, which in turn requires fewer cells per battery pack. To maintain the same power, however, higher currents are needed. This emphasizes the importance of very low cell resistance to allow unrestricted flow of current.
Chemistry variations — During recent years, several types of Li-ion batteries have emerged with only one thing in common — the catchword 'lithium'. Although strikingly similar on the outside, lithium-based batteries can vary widely. This book addresses the lithium-based batteries that are predominantly used in commercial products.
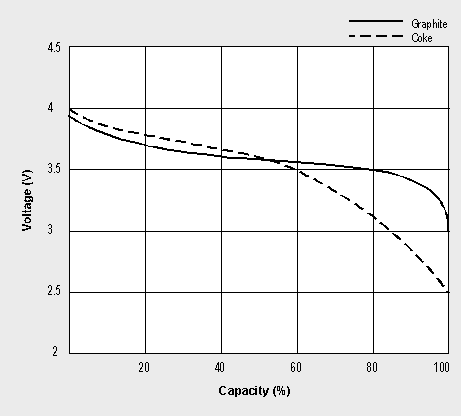
Sony’s original version of the Li-ion used coke, a product of coal, as the negative electrode. Since 1997, most Li-ions (including Sony’s) have shifted to graphite. This electrode provides a flatter discharge voltage curve than coke and offers a sharp knee bend at the end of discharge (see Figure 2-5). As a result, the graphite system delivers the stored energy by only having to discharge to 3.0V/cell, whereas the coke version must be discharged to 2.5V to get similar runtime. In addition, the graphite version is capable of delivering a higher discharge current and remains cooler during charge and discharge than the coke version.
For the positive electrode, two distinct chemistries have emerged. They are cobalt and spinel (also known as manganese). Whereas cobalt has been in use longer, spinel is inherently safer and more forgiving if abused. Small prismatic spinel packs for mobile phones may only include a thermal fuse and temperature sensor. In addition to cost savings on a simplified protection circuit, the raw material cost for spinel is lower than that of cobalt.
Figure 2-5: Li-ion discharge characteristics.
The graphite Li-ion only needs to discharge to 3.0V/cell, whereas the coke version must be discharged to 2.5V/cell to achieve similar performance.