<< Previous page INDEX Next page >>
4.5 Charging the Lithium Ion Battery
The Li-ion charger is a voltage-limiting device similar to the lead acid battery charger. The difference lies in a higher voltage per cell, tighter voltage tolerance and the absence of trickle or float charge when full charge is reached.
While the lead acid battery offers some flexibility in terms of voltage cut-off, manufacturers of Li-ion cells are very strict on setting the correct voltage. When the Li- ion was first introduced, the graphite system demanded a charge voltage limit of 4.10V/cell. Although higher voltages deliver increased energy densities, cell oxidation severely limited the service life in the early graphite cells that were charged above the 4.10V/cell threshold. This effect has been solved with chemical additives. Most commercial Li-ion cells can now be charged to 4.20V. The tolerance on all Li-ion batteries is a tight +/-0.05V/cell.
Industrial and military Li-ion batteries designed for maximum cycle life use an end-of-charge voltage threshold of about 3.90V/cell. These batteries are rated lower on the watt-hour-per-kilogram scale, but longevity takes precedence over high energy density and small size.
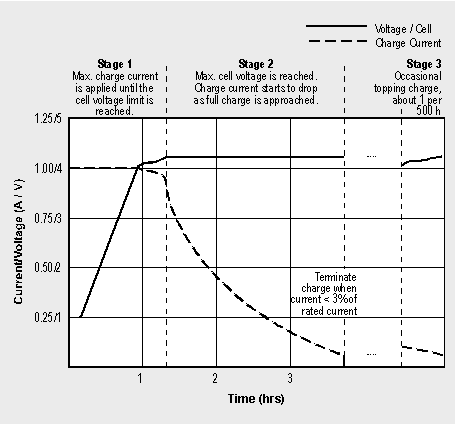
The charge time of all Li-ion batteries, when charged at a 1C initial current, is about 3 hours. The battery remains cool during charge. Full charge is attained after the voltage has reached the upper voltage threshold and the current has dropped and leveled off at about 3 percent of the nominal charge current.
Increasing the charge current on a Li-ion charger does not shorten the charge time by much. Although the voltage peak is reached quicker with higher current, the topping charge will take longer. Figure 4-5 shows the voltage and current signature of a charger as the Li-ion cell passes through stage one and two.
Some chargers claim to fast-charge a Li-ion battery in one hour or less. Such a charger eliminates stage 2 and goes directly to ‘ready’ once the voltage threshold is reached at the end of stage 1. The charge level at this point is about 70 percent. The topping charge typically takes twice as long as the initial charge.
No trickle charge is applied because the Li-ion is unable to absorb overcharge. Trickle charge could cause plating of metallic lithium, a condition that renders the cell unstable. Instead, a brief topping charge is applied to compensate for the small amount of self-discharge the battery and its protective circuit consume.
Depending on the charger and the self-discharge of the battery, a topping charge may be implemented once every 500 hours or 20 days. Typically, the charge kicks in when the open terminal voltage drops to 4.05V/cell and turns off when it reaches 4.20V/cell again.
Figure 4-5: Charge stages of a Li-ion battery.
Increasing the charge current on a Li-ion charger does not shorten the charge time by much. Although the voltage peak is reached quicker with higher current, the topping charge will take longer.What if a battery is inadvertently overcharged? Li-ion batteries are designed to operate safely within their normal operating voltage but become increasingly unstable if charged to higher voltages. On a charge voltage above 4.30V, the cell causes lithium metal plating on the anode. In addition, the cathode material becomes an oxidizing agent, loses stability and releases oxygen. Overcharging causes the cell to heat up.
Much attention has been placed on the safety of the Li-ion battery. Commercial Li-ion battery packs contain a protection circuit that prevents the cell voltage from going too high while charging. The typical safety threshold is set to 4.30V/cell. In addition, temperature sensing disconnects the charge if the internal temperature approaches 90°C (194°F). Most cells feature a mechanical pressure switch that permanently interrupts the current path if a safe pressure threshold is exceeded. Internal voltage control circuits cut off the battery at low and high voltage points.
Exceptions are made on some spinel (manganese) packs containing one or two small cells. On overcharge, this chemistry produces minimal lithium plating on the anode because most metallic lithium has been removed from the cathode during normal charging. The cathode material remains stable and does not generate oxygen unless the cell gets extremely hot.
Important: In case of rupture, leaking electrolyte or any other cause of exposure to the electrolyte, flush with water immediately. If eye exposure occurs, flush with water for 15 minutes and consult a physician immediately.